is salt solution heterogeneous or homogeneous
What other solutions have you made. Emulsions are homogeneous mixtures although they often become.

Heterogeneous And Homogeneous Mixtures Physical Science Matter Winter Holiday Christmas Christmas Science Middle School Science Activities Winter Holidays
Solutions injected into the body must.

. Chocolate chip cookies are a heterogeneous mixture. Discover the parts of a solution and see examples of the three types of solutions. The key difference between homogeneous and heterogeneous is that homogeneous materials and mixtures have the same uniform composition and properties throughout whereas heterogeneous materials and mixtures do not have either uniform composition or uniform properties.
Often it is easy to confuse a homogeneous mixture with a pure substance because they are both uniform. Heterogeneous nucleation nucleation with the nucleus at a surface is much more common than homogeneous nucleation. Any chemical solution or alloy is a homogeneous mixture.
The amount of salt in the salt water can vary from one sample to another. Many common chemicals are homogeneous mixtures. The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample.
Examples of homogeneous solutions are a cup of coffee perfume salt or sugar in water etc and the examples of heterogeneous solutions are a solution of oil and water a solution of sand and water a solution of chalk powder and water etc. Nitrogen Pure substance E 5. As a monovalent ion salt Cs salt has the same potential to form a WIS electrolyte.
Mixtures can be either homogeneous or heterogeneous. The saltwater mentioned above is homogeneous due to the even distribution of the dissolved salt throughout the entire sample of saltwater. Oxygen Pure substance E 15.
Other examples of homogeneous mixtures include air maple syrup gasoline and a solution of salt in water. Solute concentrations are particularly important when solutions are injected into the body. This type of mixture is also called the solution and is made by the solute and solvent.
Homogeneous and heterogeneous mixtures. Salt water Mixture 3. The substances having the same homogeneous phase in all properties are called completely miscible in one another but if the substances are insoluble in.
Soda is considered a heterogeneous mixture. Examples of solutions include sugar water and powdered drink mix in water while alloys include sterling silver and bronze. Solutes in body cell fluids and blood serum give these solutions an osmotic pressure of approximately 77 atm.
Classify the following types of matter as either homogeneous or heterogeneous. While the sugar water and flavorings may form a chemical solution the. Chocolate chip ice cream Mixture 4.
Osmosis can also affect animal cells. Here the U-233 is progressively removed and transferred to the primary circuit. If you take a bite from a cookie you may not get the same number of chips as you get in another bite.
Examples include vodka. Solid liquid and gas. Isopropyl alcohol Pure substance C 16.
They have visible boundaries of separation between the constituents. The exception would be solutions that contain another phase of matter. A homogeneous equilibrium is one in which all of the reactants and products are present in a single solution by definition a homogeneous mixture.
A cucumber placed in a concentrated salt solution loses water by osmosis and absorbs some salt to become a pickle. Refer to the associated activity Messin. Most of the time it is a liquid while the solvents are present in the dominant quantity.
The Cs-based WIS electrolyte has positive effects on the electrochemical performance of SCs including increasing capacity suppressing self-discharge and improving charge-discharge efficiency. The difference is that the composition of the substance is always the same. Homogeneous Mixtures are those in which participants can not be distinguished and also cant be identified.
B A commercial sports drink is a homogeneous mixture because its composition is uniform throughout. It probably is and that is one characteristic of a solution that it is homogeneous or that it is uniform throughout. CsF with a saturated solubility of 395 m in water has been used to prepare WIS electrolytes for SC 144.
Two-fluid or heterogeneous MSRs would have fertile salt containing thorium in a second loop separate from the fuel salt containing fissile uranium or plutonium and could operate as a breeder reactor MSBR. The solutes are always present in low quantity. A homogeneous mixture is a mixture throughout the solution in which the composition is uniform.
For example in the nucleation of ice from supercooled water droplets purifying the water to remove all or almost all impurities results in water droplets that freeze below around 35 C whereas water that contains impurities may freeze at 5 C or warmer. Mixture of sand and common salt Mixture of sand and water etc. For example you can make a homogeneous solution of sugar and water but if there are crystals in the solution it becomes a heterogeneous mixture.
Reactions between solutes in liquid solutions belong to one type of homogeneous equilibria. Modification of work by John Mayer. A heterogeneous mixture has physically distinct components.
It contains water sugar and carbon dioxide which forms bubbles. Alloys Solution of salts in water etc. However graphite degradation from neutron flux limits the useful life of the reactor core with the fuel.
Salt water mud pies bubbles etc Solutions are types of mixtures but mixtures can also be heterogeneous where you can see the different ingredients separated out. Carbon dioxide Pure. Salt and pepper form a heterogeneous mixture.
They have no visible boundaries of separation between the constituents. A mixture in which constituents are distributed uniformly such as salt in water is called homogeneous whereas a mixture whose constituents are clearly separate from one another such as sand in water it is called heterogeneous. A scientific solution is defined as two or more substances in a homogenous mixture.
A Oil and vinegar salad dressing is a heterogeneous mixture because its composition is not uniform throughout. Chemical solutions are usually homogeneous mixtures. Carbonated soft drink w bubbles heterogeneous 9.
Ie dissolving sugar in water cannot be separated. In addition uniform mixture is another term for homogenous mixture. Homogeneous and heterogeneous are two different words that we can distinguish by the context in.

Frank Icse Solutions For Class 9 Chemistry Elements Compounds And Mixtures A Plus Topper Separationofmixturesc Compounds And Mixtures Chemistry Solutions

Heterogeneous And Homogeneous Mixtures Editable Powerpoint Editable Powerpoint Homogeneous Mixture Powerpoint

A Scheme For Classifying Matter Teaching Chemistry Science Lessons Chemistry

Pure Substances And Mixtures Pure Products Elements Compounds And Mixtures Compounds And Mixtures

Let S Mention The Difference Between A Solution And A Heterogeneous Mixture A Solution Is A Comb Chemical Science Solutions And Mixtures Heterogeneous Mixture

Mixtures Homogeneous Heterogeneous And Solutions Power Point Types Of Mixtures Heterogeneous Mixture Chemical And Physical Changes

Mixtures And Pure Substances Heterogeneous Mixture Pure Products Mixtures

Mixtures And Solutions Matter Science Solubility Examples Of Mixtures

Pin Di Carrie Flanagan Su School Stuff Tavola Periodica

What S The Difference Between Heterogeneous And Homogeneous Mixtures Examples Of Mixtures Heterogeneous Mixture Chemistry

Is Matter Around Us Pure School Help By Gunjan Pure Products School Help Homogeneous Mixture

Physical Science Lessons 5th Grade Science Middle School Science Experiments

Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Middle School Science Resources

Heterogeneous And Homogeneous Mixtures Write And Draw Worksheet School Science Experiments Middle School Science Experiments Teaching Chemistry
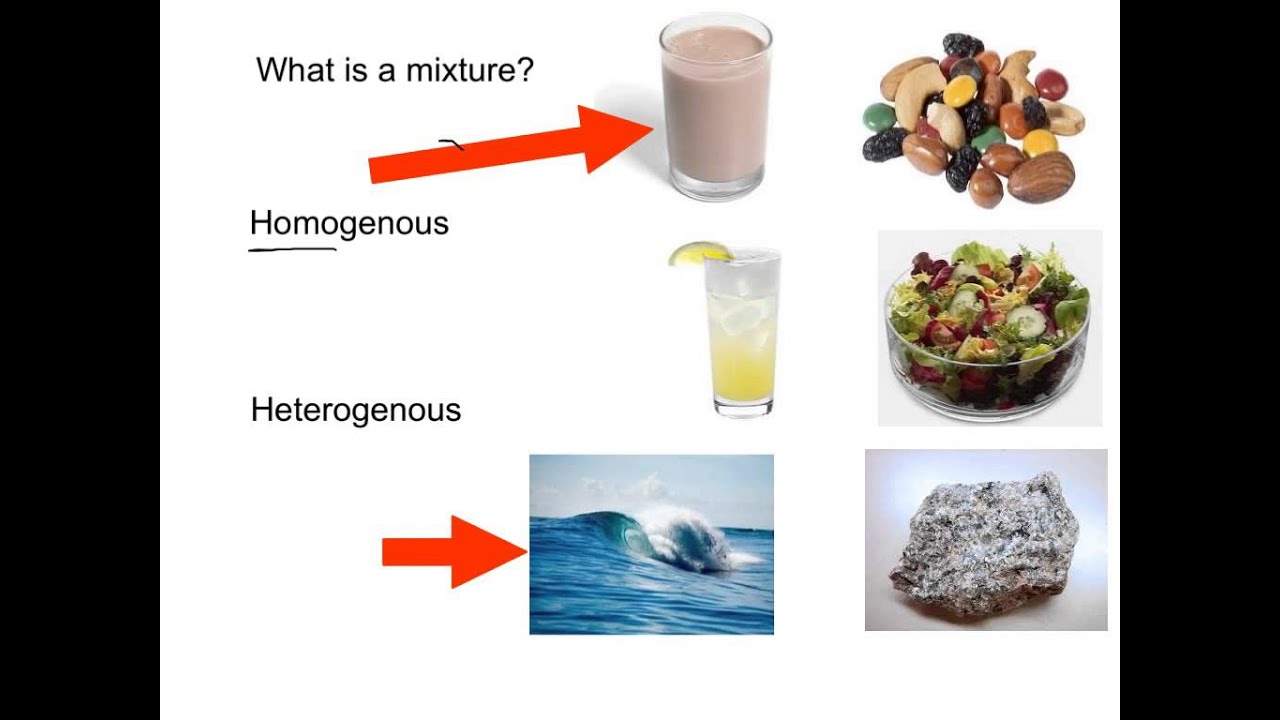
Pin On Middle School Chemistry

New Ap General Chemistry Video Playlist Youtube Chemistry Heterogeneous Mixture Organic Chemistry Tutor

Classification Of Matter Ultimate Card Sort For Pure Substances And Mixtures Sorting Cards Creative Teaching Teacher Preparation

Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Middle School Science Resources

Examples Of Pure Substances Pure Products How To Make Tea Types Of Mixtures
0 Response to "is salt solution heterogeneous or homogeneous"
Post a Comment